In the prior art, there are methods and devices with which carbon dioxide can be extracted from air or gas mixtures. What these methods have in common is that a high level of procedural effort and high energy inputs are required in order, on the one hand, to carry out a largely complete extraction of carbon dioxide and, on the other hand, to obtain the extracted carbon dioxide as a pure gas. A complete extraction is necessary, for example, if a biogas or sewage gas is to be purified in order to obtain pure methane. On the other hand, carbon dioxide can only be used economically if it is present in a purity of > 99% by volume. The state-of-the-art plant technologies are geared towards high volume throughputs and require a high initial investment and considerable maintenance costs. In addition, some of the processes are ecologically questionable (e.g. when using monetholamine or its derivatives) and cannot be carried out without product loss (e.g. methane slip). Therefore, in spite of a considerable need, these methods have not yet been widely used.
A process has been developed with which it is possible, by means of a biogenic substance dissolved in water, to completely extract carbon dioxide from any air / gas mixture in a highly effective manner and to bind it without pressure. The carbon dioxide bound in the aqueous acceptor medium can be selectively released again by means of an electrophoretic process, as a result of which it is obtained in pure form. The acceptor solution regenerated in this way can then be used for a renewed extraction of carbon dioxide. Thus, the process can be operated continuously, without product loss and ecologically harmless, and an air-/gas mixture that is free of carbon dioxide as well as a pure gas of carbon dioxide are obtained.
 In the processes for extracting carbon dioxide that exist in the prior art, there are essentially three types of process: 1. gas scrubbing process, 2. adsorption process and 3. membrane process. In the washing process, so-called amine washing is widespread in which in aqueous solutions containing monetholamine or its derivatives are used, which act as acceptor for carbon dioxide. Desorption is achieved by increasing the temperature of the acceptor solution to 120 ° C. During the process, a small part of the monetholamine escapes. Another aqueous process is pressure swing scrubbing. For this purpose, the entire gas flow must be compressed to 4 - 6 bar while mixing with water. The carbon dioxide dissolved in water is then desorbed by applying a negative pressure. In the adsorption process, carbon dioxide is adsorbed onto solids. For regeneration, these must be heated, which is also done under vacuum. Gas separation membranes are used in the membrane process. A retention of carbon dioxide can be achieved under high pressure. However, it is only possible to obtain pure gases with this process with further process effort. What the processes have in common is that enormous energy losses occur as a result of the fact that a large part of the required energy is lost in the form of thermal energy or pressure energy.
In the processes for extracting carbon dioxide that exist in the prior art, there are essentially three types of process: 1. gas scrubbing process, 2. adsorption process and 3. membrane process. In the washing process, so-called amine washing is widespread in which in aqueous solutions containing monetholamine or its derivatives are used, which act as acceptor for carbon dioxide. Desorption is achieved by increasing the temperature of the acceptor solution to 120 ° C. During the process, a small part of the monetholamine escapes. Another aqueous process is pressure swing scrubbing. For this purpose, the entire gas flow must be compressed to 4 - 6 bar while mixing with water. The carbon dioxide dissolved in water is then desorbed by applying a negative pressure. In the adsorption process, carbon dioxide is adsorbed onto solids. For regeneration, these must be heated, which is also done under vacuum. Gas separation membranes are used in the membrane process. A retention of carbon dioxide can be achieved under high pressure. However, it is only possible to obtain pure gases with this process with further process effort. What the processes have in common is that enormous energy losses occur as a result of the fact that a large part of the required energy is lost in the form of thermal energy or pressure energy.
A process was developed in which a physiological amino acid is used to electrostatically bind the water-soluble form of carbon dioxide (carbonate anions). This reaction takes place spontaneously at temperatures between 0 and 99 ° C. This makes it possible to bind more than 120g / carbon dioxide in one liter of the aqueous acceptor solution without pressure and to transport it therein. Following the binding, selective release can take place in an electrodialysis device. The carbonate anions are transported electrophoretically through a membrane suitable for selective material transport. After passing through the membrane, the carbonate anions are bound in an aqueous transport medium and passed outside the electrodialysis unit into an outgassing unit, where outgassing occurs spontaneously in the form of carbon dioxide. The acceptor liquid is regenerated while passing through the electrodialysis unit and can be used again for the extraction of carbon dioxide.
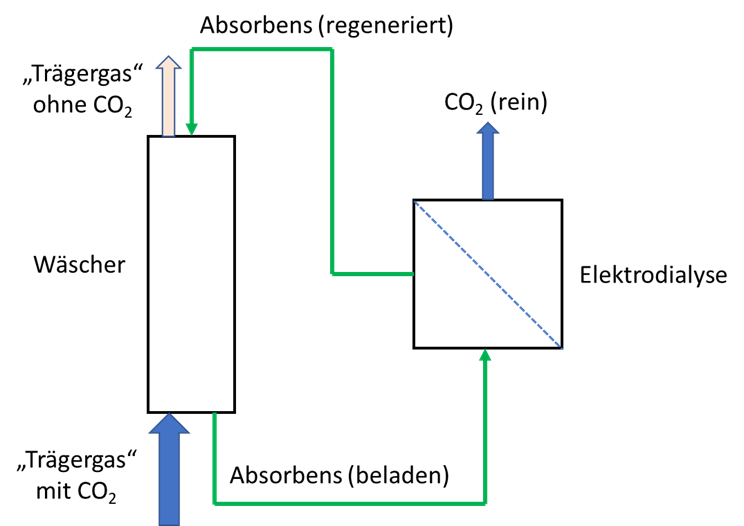 This method and the way in which the method is carried out result in significant advantages compared to the methods presented from the prior art. The process has no ecological concern and is carried out with a physiologically occurring compound that is made from renewable raw materials. There is no loss of this compound, as could be shown in long-term operation. The extraction of carbon dioxide can take place with a gas scrubber available in the prior art and is therefore scalable as required. The carbon dioxide dissolved in the form of carbonate anions is separated electrokinetically, so that the energy input is used almost exclusively for the separation. This enables an energy efficiency of > 80% to be achieved. As a by-product of the energy input, pure gases from hydrogen and oxygen, which are created in the electrolyte chambers by electrolysis, are obtained. Due to the selective transport of carbon anions by means of the method, a pure gas of carbon dioxide is immediately available.
This method and the way in which the method is carried out result in significant advantages compared to the methods presented from the prior art. The process has no ecological concern and is carried out with a physiologically occurring compound that is made from renewable raw materials. There is no loss of this compound, as could be shown in long-term operation. The extraction of carbon dioxide can take place with a gas scrubber available in the prior art and is therefore scalable as required. The carbon dioxide dissolved in the form of carbonate anions is separated electrokinetically, so that the energy input is used almost exclusively for the separation. This enables an energy efficiency of > 80% to be achieved. As a by-product of the energy input, pure gases from hydrogen and oxygen, which are created in the electrolyte chambers by electrolysis, are obtained. Due to the selective transport of carbon anions by means of the method, a pure gas of carbon dioxide is immediately available.

